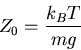Next: About this document ...
29:61 General Astronomy
Fall 2004
Lecture 1 ...August 23,2004
The Earth's Atmosphere and the Boundary of Space
Just the facts, Ma'am
 Atmospheric pressure at sea level:
Atmospheric pressure at sea level:
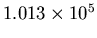 Newtons/m
Newtons/m
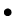 Change in atmospheric pressure associated with increase in altitude
Change in atmospheric pressure associated with increase in altitude 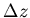
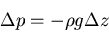 |
(1) |
where 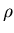 is the gas density (kilograms/m
is the gas density (kilograms/m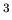 ),
), 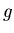 acceleration due to gravity at Earth's surface (9.8 m/sec
acceleration due to gravity at Earth's surface (9.8 m/sec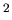 ).
).
 Perfect gas law
Perfect gas law
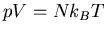 |
|
|
(2) |
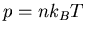 |
|
|
(3) |
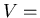 volume of gas,
volume of gas, 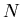 total number of particles (atoms and/or molecules) in the gas,
total number of particles (atoms and/or molecules) in the gas, 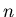 number density of atoms and/or molecules (units are particles/m
number density of atoms and/or molecules (units are particles/m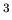 ),
), 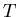 is the temperature (degrees Kelvin),
is the temperature (degrees Kelvin),
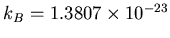 J/K.
J/K.
 Differential form of change in atmospheric pressure with altitude
Differential form of change in atmospheric pressure with altitude
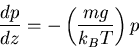 |
(4) |
where 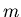 mass of atom or molecule composing the atmosphere.
mass of atom or molecule composing the atmosphere.
 Pressure as function of height in an isothermal atmosphere
Pressure as function of height in an isothermal atmosphere
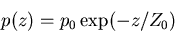 |
(5) |
 Isothermal pressure scale height
Isothermal pressure scale height
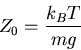 |
(6) |



Next: About this document ...
Steve Spangler
2004-08-23
![]() Change in atmospheric pressure associated with increase in altitude
Change in atmospheric pressure associated with increase in altitude ![]()
![]() Perfect gas law
Perfect gas law
![]() Differential form of change in atmospheric pressure with altitude
Differential form of change in atmospheric pressure with altitude
![]() Pressure as function of height in an isothermal atmosphere
Pressure as function of height in an isothermal atmosphere
![]() Isothermal pressure scale height
Isothermal pressure scale height